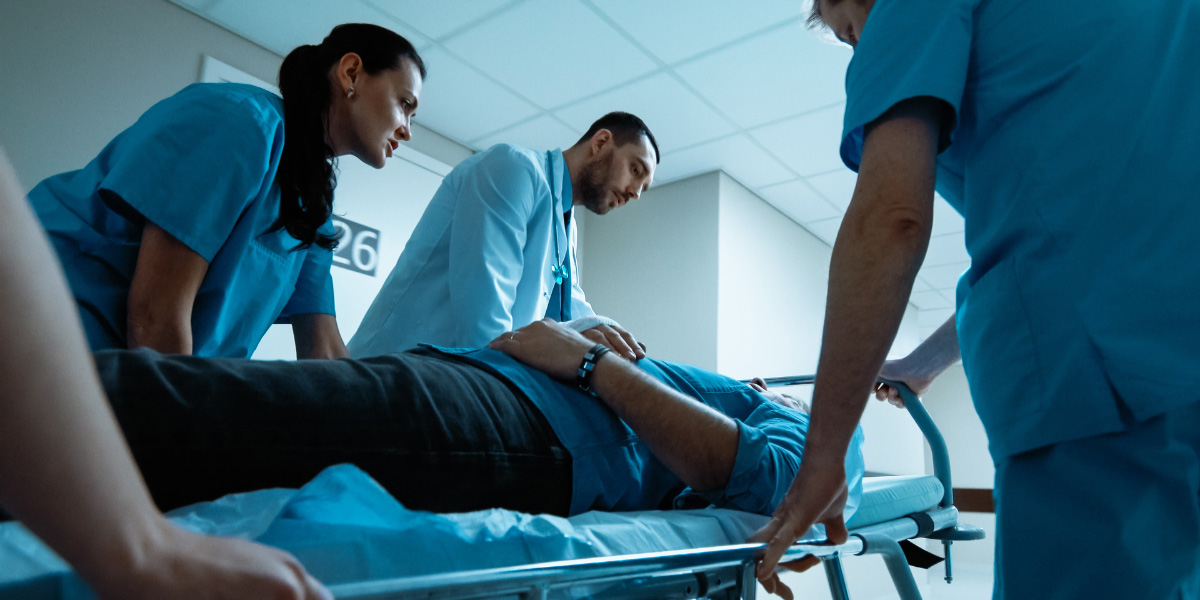
High-Volume Emergency Department Boosts Efficiency by Adding Scribes
A busy Emergency Department decided in early 2023 to explore solutions to address labor shortages and rising costs. Partnering with ScribeAmerica, they initiated 1:1 scribe coverage for all providers in the department.
READ STORY

Rural Health Center Winding Waters Makes Big Impact by Adding Speke AI Scribe
Serving nearly 2,200 citizens in Enterprise, Oregon, the non-profit FQHC reached out to ScribeAmerica to help them make a difference.
READ STORY

More Pets, Less Vets: Battling Veterinarian Burnout in a Post-COVID World
Veterinarian burnout has reached its boiling point. Now, animal care providers are searching for solutions to improve ROI and generate a healthy work-life balance for their employees.
READ STORY
KLAS Spotlight: Speke
The KLAS Emerging Technology Spotlight report examines customer experience and overall satisfaction using Speke, ScribeAmerica’s Ambient AI Scribe. Customers gave Speke high performance grades in all categories, and 100% said they would buy again. Additionally, respondents said Speke helped decrease provider burnout and increased capacity and available time with patients.
READ STORY

JHMHP: Hospitalist scribe study – improved satisfaction, productivity and ROI
Pairing a medical scribe with an admitting hospitalist
physician led to increased clinician satisfaction, decreased
burnout symptoms, and improved productivity. The
financial value was demonstrated by a generous ROI.
READ STORY

ScribeAmerica Update: Speke In-App Feedback
Provide quick and easy feedback to help improve the quality of your documentation
READ STORY

Big Response in the Big Apple
Speke supports frontline provider documentation during the early COVID-19 response at New York's Central Park Field Hospital
READ STORY